Ophthalmologist Camiel Boon has anambitious goal: to find a cure for genetic eye diseases such as X-linked retinoschisis. A recently acquired €800,000 VIDI grant should give him and his group at Amsterdam Neuroscience a head start. “We’ll begin with a good animal model plus a ‘retina-in-a-dish’, to advance our knowledge on this invalidating disease and to develop cutting-edge genetic therapy.”
Read More
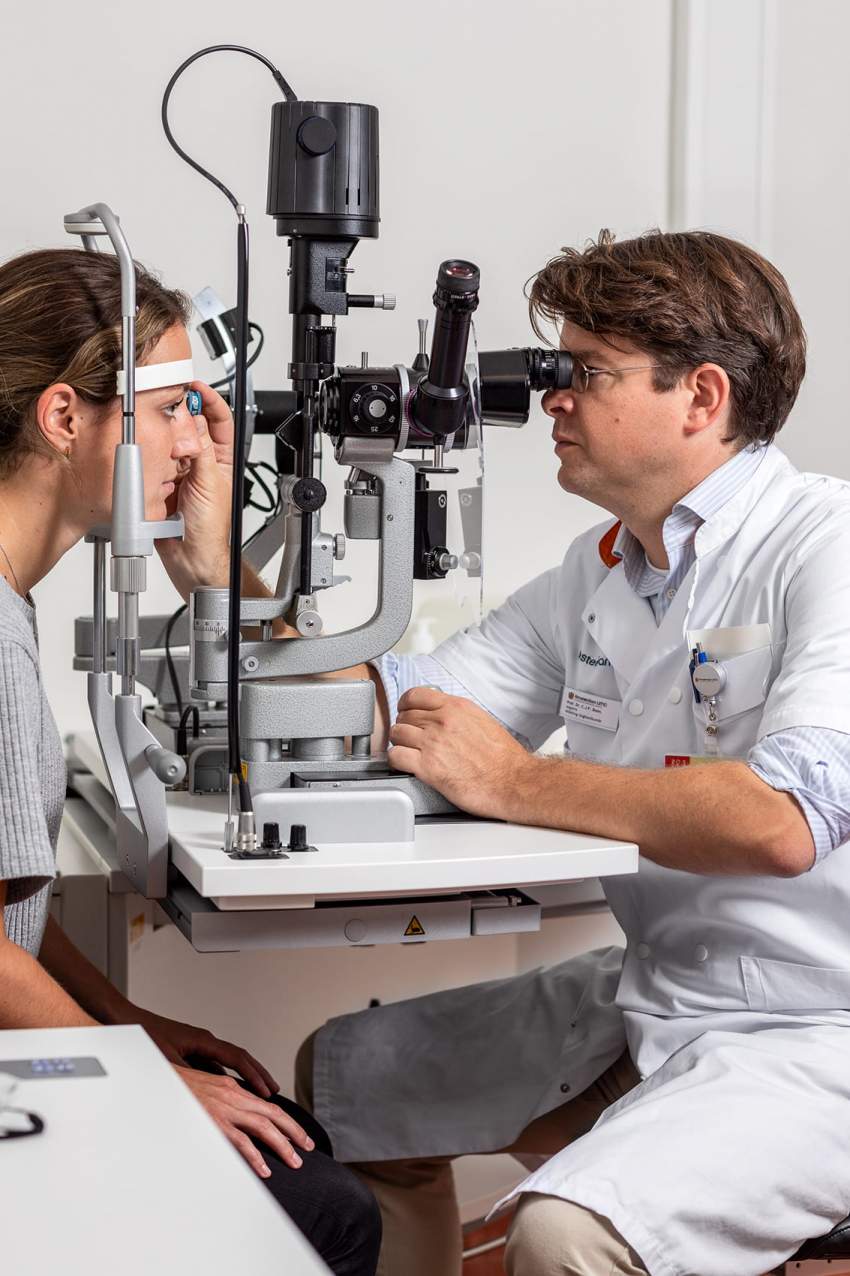
Both as a clinician and a scientist, who has specialized in diseases of the retina and retinal surgery and whose research is focused on hereditary retinal dystrophies, Camiel Boon is highly fascinated by the beauty of the eye, as well as by the impact of ocular pathology. “Just looking at the retina and knowing which genetic and neurological processes are going on there, quite literally in the blink of an eye, evokes sheer fascination,” says the Professor of Ophthalmology and Clinical Ophthalmogenetics. “And then seeing patients every week, whose lives are so severely impacted by diminishing sight, or even blindness, really motivates me to make a difference there. Which is why we are taking part in several first-ever Phase 2-3 clinical trials for gene therapy and RNA therapeutics in these severe retinal degenerations.”
The €800,000VIDI grant that Boon was awarded last year was therefore more than welcome. “We’re going to use it to work on X-linked retinoschisis, which is the third most common genetic retinal pathology after retinitis pigmentosa and Stargardt disease,” says Boon. “A defect on the X-chromosome causes the layers of the retina to split, and the problems generally start shortly after birth, with significant loss of vision. It generally takes a long period before this leads to complete blindness, so there is also a relatively long period for potential intervention.”
Two-pronged attack
Boon intends to start his ‘attack’ on the disease through two approaches. “First of all, we are developing a good animal model to further study the specific pathology of retinoschisis. Secondly, we want to develop a ‘retina-in-a-dish’ organoid model. These patient-derived cultured retinal cells will not only advance our knowledge of the disease, they will also potentially provide opportunities to test new medicines. For this, we will be working closely with the lab of Arthur Bergen, professor in complex ophthalmogenetics at Amsterdam UMC.”
Boon specifically aims at genetic techniques to fight retinoschisis in these disease models. “By introducing a healthy gene, which is delivered via a harmless virus to the retina, it might also be possible to treat other genetic disorders in the retina. Currently, the drug voretigene neparvovec (Luxturna®) is the first-ever FDA- and EMA-approved gene therapy, in this case for a rare retinal disease called Leber congenital amaurosis. It specifically replaces the defective RPE65 gene in these patients, and we recently treated the first patient in the Amsterdam UMC with this new therapy. But several other gene therapies are in advanced stages of clinical testing. Our clinical trial center is a European ‘clinical trial hub’, and we have included several patients from other European countries into these groundbreaking treatment trials. However, there are many more genetic causes such as those in X-linked retinoschisis for which these treatments still need to be developed, and at Amsterdam UMC, we’re taking an active part in this.”
Logical field for pioneering
“Ophthalmology is undoubtedly a logical field for pioneering when it comes to gene therapy,” Boon states. “One could see the eye as a well-defined ‘porch’ of the brain. The light that enters our eyes sets off a loop of processes, the so-called visual cycle. It transforms light into electrical signals that eventually reach the visual cortex in the brain. This whole ‘loop’ occurs within the well-defined boundaries of the retinal photoreceptors – the cones and rods – and takes less than a split-second. If a genetic defect blocks this loop, the system’s resilience can be restored by administering gene therapy, targeted precisely at these cells within the eye. As we can deliver these innovative therapies to the relatively closed compartment of the eye, using meticulous microsurgical techniques, there is basically little risk of side effects in other parts of the body. Which is a comforting thought in such an innovative field as gene therapy.”
Working towards a cure
At the end of his five-year VIDI grant, Boon hopes to have new leads to continue working on actual therapies for genetic eye diseases such as X-linked retinoschisis. “They don’t necessarily have to be gene replacement therapies,” he says. “They could also be through new techniques like CRISPR/Cas, that can ‘clip’ a defective gene out of the affected cells, replacing it with a healthy version, or RNA-based treatments that attack the faulty genetic process a little bit further down the line. Whatever works to save sight – that’s all that matters. As a clinician-scientist, I find it exciting to work in these pioneering times of precision medicine to reach this goal.”
Photography: Marieke de Lorijn